The subject of today's post is the gamma spectrum of uranium glass, which I happened to come across. Probably everyone involved in gamma spectrometry has had the chance to measure uranium containing glassware or "Fiestaware" - a fascinating, or maybe insane, piece of history, when kitchen glass or porcelain was colored with uranium compounds.
Uranium glass, often known for its striking green or yellow hue is a type of glass that contains small amounts of uranium, typically added in the form of oxide. Under natural light, it often appears like any other colored glass, but under UV light emits a vibrant, eerie glow, usually in shades of green (see below). This fluorescence is just due to the presence of uranium ions, which absorb UV light and re-emit it as visible light.
In terms of its physical characteristics, uranium glass is similar to other glass types. It is hard, brittle, and can be crafted into various forms such as bowls, vases, and ornamental objects. However, the presence of uranium slightly increases the glass's density and refractive index, leading to a characteristic shine and color depth.
A Brief History
The history of uranium glass began in our country and dates back to at least the early 19th century, with the first known production attributed to Czech glassmaker Josef Riedel in the 1830s. The use of uranium in glass became more widespread across Europe during the Victorian era, especially for decorative objects. However, production of uranium glass declined significantly after World War II due to concerns over radioactivity and regulations governing the use of uranium.
Chemical Composition
The uranium in uranium glass is typically added in the form of uranium dioxide UO₂ or uranyl nitrate UO₂(NO₃)₂, both of which dissolve into the molten glass during the manufacturing process. Uranium content usually varies between 0.1% and 2% by weight. In modern applications, due to regulatory concerns, the uranium concentration tends to be on the lower end of the spectrum.
Historically, uranium glassmakers used naturally occurring uranium, which contains about 0.7% of the fissile isotope uranium-235. However, as the nuclear industry expanded and enriched uranium became prioritized for reactors and weapons, depleted uranium became more commonly available for non-nuclear applications. Today, virtually all uranium glass production uses depleted uranium, which contains less than 0.3% uranium-235.
Health and Safety Concerns
Though uranium is radioactive, the health risks associated with uranium glass are generally considered low. The uranium content in the glass is typically insufficient to pose significant hazards from external exposure, as uranium primarily emits alpha particles, which cannot penetrate the glass or human skin. However, the main concern arises from long-term inhalation or ingestion of uranium glass dust, which could occur if the glass is chipped, broken, or ground.
Gamma spectrum
To obtain a decent gamma spectrum, the signal acquisition from the spectrometer took nearly 26 hours. After just over a day, I was rewarded with a rather nice, low-noise spectrum of the uranium glass sample. It revealed many peaks of known radioisotopes, but also a few that are quite difficult for me to interpret, or could be ambiguous given the limitations of low-resolution gamma spectrometry.
| Sample | Uranium glass |
| Gamma spectrometer | Scintillix SCGS-01 |
| Scintillation probe | NaI(Tl) 3" |
| Integration time | 92660 sec. (25.7 h) |
| Background correction | No |
| Shield | Lead |
| Software | Theremino v.7.2 |
The radionuclides that can be easily matched to their corresponding peaks include U-235, with peaks at 143.8 keV and 185.7 keV, as well as Pa-234m, with peaks at 766.4 keV and 1000.9 keV. A small peak at 511 keV corresponds to the annihilation peak. With slightly less confidence, I assigned U-238 peaks at 112.8 keV and 258.3 keV. However, the peaks at 34, 67, 80, and 96 keV are a complete mystery to me. Some of them could be due to lead fluorescence (perhaps 80 and 96 keV?)
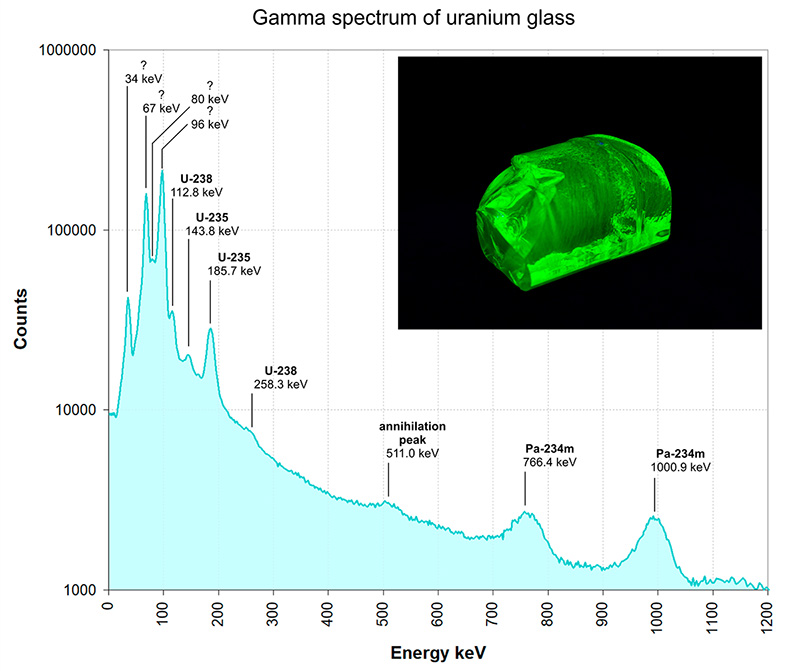
Gamma spectrum of uranium glass.The Y-axis is in log scale to better show the low intensity peaks above 200 keV.

